Karst Research Institute ZRC SAZU, Postojna, Slovenia
Abstract
The weathered parts of carbonate bedrock on cave walls are a consequence of its incomplete chemical dissolution. The phenomenon is expressed in parts of the caves where walls are in contact with clastic fluvial sediments, wetted by percolation water or wetted by condensation water, and not rinsed by flowing or dripping water. The temperature in the cave is not an important parameter of weathered zone formation. Incomplete dissolution is characteristic both of Alpine and of Mediterranean caves. Limestone or dolomite are dissolved by corrosive moisture; the dissolution is distinctly selective and it go as on at intervals depending on inflow of new aggressive water. The weathered zone of limestone or dolomite is almost identical to the parent rocks in its chemical and mineral composition yet it is much more porous. During chemical weathering the amount of Mg, Sr and U is decreased, these components being leached out of limestone and dolomite. The amount of insoluble residue is usually higher in weathered limestones and in some other cases in fresh limestones which is not very common but it may occur.
Keywords by authors: weathering, limestone, dolomite, cave, incomplete dissolution, selective corrosion, soluble residue
Introduction
The appearance of thick, soft zones of an unknown white, silt- or clay-like substance on the walls of cave passages (Fig. 1), looking very much like moon-milk precipitated on cave walls, was the main interest of this research work. Investigated by speleological, geological and chemical research methods, it was realised that these materials are a "soluble" residue of limestone and dolomite solution.
Fig. 1. Weathered cave walls in Martinska jama, SW of Slovenia. Cave was formed in Cretaceous limestone.
On the cave walls thick zones of weathered limestone or dolomite remain when the solution process ends. This usually happens when there is no more inflow of aggressive water or when flowing water no longer transports the carbonate weathering products.
Carbonate rock do not dissolve immediately; and this signifies that they are not carried away completely from their primary place in ionic form, but that the disintegrated particles may remain on the cave passage walls. An incomplete dissolution may just prepare the carbonate rock for the mechanical transport of its particles by the flow of water.
Combining the field investigative work with different research methods, I wanted to answer the questions:
- Are we actually witnessing the carbonate rock weathering or just the precipitation of secondary minerals?
- In what way the carbonate rock structure influences dissolution?
- Which factors condition the depth of the weathered zone in carbonate rock?
- In which cases does the weathered rock remain on the channel wall?
A few millimeters thick weathered layer may be noticed very often on cave passage walls. It occurs in fissures, as well as on the rock surface where it is reflected in its roughness. Thicker zones of weathered bedrock, however, are much rarer, especially in larger spaces.
In the cases I have been investigating, dissolution advances well in depth, but it is, however, an incomplete dissolution. Its operation leaves behind a porous sponge-like weathered zone. The dissolution does not favor only the open cracks but also the various structures present in the rock; it dissolves smaller grains, the borders between grains, etc.
The appearance of incomplete dissolution occurs in cave passages, as well as on the earth's surface. Carbonate rock covered by clastic sediments or soil will usually weather under them also. On the surface of a rock covered by alluvium or soil, the so-called subterranean rocky features may get formed due to the flow of water along their contact areas. Their formation as well as the shaping of their surface is, besides the manner of water flow, decided also by the bedrock composition, by the layer graduation structuring and the extent of destruction (Slabe 1999). The processes of chemical reaction between carbonate rock and deposited sediment was described by Renault (1968), who stated that the acid saturated clay in contact with the dolomite absorbs Ca2+ from it and that dolomite thus becomes soft.
In the cases I have investigated, the materials which were in contact with each other were carbonate rock and the alluvium of non-carbonate composition and origin. I did not notice any kind of chemical reactions between them (in the sense of data represented by Pezdič et al. 1998), at least not on the level of field research as well as when considering their mineral composition.
Sometimes, however, we may come across cases when the carbonate rock in contact with clastic sediments does not display visible signs of being weathered (Mihevc 1996). The author describes scallops on the cave wall which are entirely preserved, although they were in the direct contact with the cave clastic sediments.
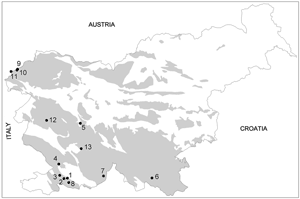
Weathered zones of carbonate bedrock may appear in caves of different geographical position and karst type; in Slovenia for example in Alpine and Dinaric karst caves, where different speleogenesis containing limestone and dolomite of different genesis and ages is presented. Research on weathered limestones and dolomites were done in the caves developed in the Upper Triassic limestone and dolomite, Lower Jurassic dolomite and limestone, in different Cretaceous limestones and dolomites and in Paleocene limestone of different genesis and textures. Case studies were done in caves Pečina v Borštu, Martinska jama, Krempljak, Jama II na Prevali, Turkova jama, Remergrund II, Spodmol na Ždroclah, Polina peč, Črne lsko Brezno, Čehi II, Renejevo brezno, Velika ledena jama v Paradani and Jama pod Pečno rebrijo (Fig. 2).
Fig. 2. Location of studied caves in Slovenia. Weathered zones of carbonate rocks may appear in caves of different geographical position, karst type and in limestone and dolomite of different origin and age. Legend: 1 - Pečina v Borštu, 2 - Martinska jama, 3 - Krempljak, 4 - Jama II na Prevali, 5 - Turkova jama, 6 - Remergrund II, 7- Spodmol na Ždroclah, 8 - Polina peč, 9 - Črnelsko brezno, 10 - Cave Čehi 2, 11 - Renejevo brezno, 12 - Velika ledena jama v Paradani, 13 - Jama pod Pečno rebrijo.
Let me emphasise that the cases I am describing, are all examples of in situ limestone and dolomite weathering and that phantom rocks with the altered chemical composition were not formed (Vergari and Qinif 1997, Kauffmann et al. 1999), but that what appeared as their residue was a porous and discoloured primary rock skeleton.
Research methods and results
Different research methods were used to find out what is going on during the weathering of limestone and dolomite in a cave environment. First the field work was done by mapping the passages where weathered cave walls are present. Also the temperature was measured and in one case the in situ pH of weathered limestone (in Pečina v Borštu cave). Samples were analyzed by chemical methods such as complexometry, EDS analysis on SEM and Ion Beam Analysis, then by x-ray powder diffraction method, in thin-sections, in cross sections of the samples by computer scanner and under the SEM.
The weathered zone of limestone and dolomite is soft when it is wet and solid when dry. The surface of a weathered cave wall retains all the structures and textures of carbonate rock, such as different laminations, fossils (Fig. 3), calcite veins; as micrite or sparite grains which seen as micro-roughness of the wall surface. On some weathered walls the beginning of the boxwork (Palmer 1981) formation is noticed. The thickness of the weathered zone varies from less than a millimetre to several centimetres. Weathered cave walls are usually covered by a brown flowstone crust unless there is direct contact with clastic fine-grained sediments where the weathered surface is uncovered.
Fig. 3. Weathered shells in Upper Cretaceous limestone, from Pečina v Borštu, SW Slovenia.
It is quite usual to find the thicker parts of the weathered rock predominantly on walls that have been or still are in contact with clastic fluvial sediments. Thicker zones of the weathered rock may come into existence also in cases where percolation water trickles along the wall. In both cases, that is under clastic sediments and when water trickles along the wall, the water is being pulled into the interior by capillary forces and along the interconnected pores and fissures. The same action takes place with condensed moisture.
In cross sections of samples the transition from fresh rock into weathered rock is quite well seen. The dissolution is progressing into the rock along the open fissures (Fig. 4) and along the invisible micro-porosity; we are unable to view the latter, neither in cross sections nor in thin sections. During weathering, the rock first of all loses some of its colour. Then the fading gradually increases, which leads to a state of complete discoloration, at which point the rock’s residue becomes white. Through the weathering, that is, through dissolution of individual parts of the rock with carbonic acid, the previously solid compact rock becomes more and more porous. And this does not occur, as we may have expected, only along fissures but also in non-fissured parts (Fig. 4). The dissolution advances into the rock's interior along the chosen structures and leaves in its wake ever larger and more interconnected pores. If the dissolution continues, the sponge-like structure may fall apart and the outer part of what was once a solid rock becomes clay-like and completely soft. The collapsing particles are, with regard to the primary rock structure, of the size order of silt or clay.
Fig. 4. Progressing of dissolution is faster along open fissures and it is stopped by calcite veins. Paleocene limestone from Jama II na Prevali. Width of the sample is 2,5 cm.
Chemical analysis results demonstrated that the amount of Mg, Sr and U in the weathered zone of carbonate rock consistently decreases with weathering. Thus it became evident that during the weathering they are actually disappearing. Mg is leached from the calcite as well as from the dolomite crystal lattice. To where and in what manner remains unknown to me.
The mineral composition of the fresh or weathered parts of carbonate rock does not differ significantly. The amount of insoluble residue is sometimes higher in the weathered and sometimes in the fresh part. The conclusion one may draw from the analyses is that limestones and dolomites in the course of weathering become purer and simultaneously lose their mechanical solidity. By means of mineralogical investigation of the clastic allochthonous sediments I discovered that they do not react chemically with the weathered rock; however they do contribute the moisture required for dissolution. Yet, we do not know whether the deposited sediment might have contained minerals, which would chemically react with the rock on the passage wall and which are not present any more.
Discussion
According to Dreybrodt (1988) the overall dissolution rate is determined by the dissolution on the crystal's surface, by the transportation of ions through the border layer and by the speed of the conversion CO2 + H2O = H+ + HCO3- as well by the lithological parameters of the carbonate rocks. Weathered zones are the result of incomplete carbonate rock dissolution. Thick zones of the weathered bedrock are rare, especially on the larger surfaces. At first sight the most weathered walls appear to be those wetted by percolating water and which are in contact with fluvial sediments, and walls which are subjected to condensation corrosion.
During the selective dissolution of individual parts the once compact carbonate rock becomes more and more porous, not only along the cracks, but also along various structures. From analyses of cross sections under SEM, it is quite evident that what is happening is not a case of secondary minerals' precipitation but of dissolving carbonate rock, which increases the porosity of the rock.
Field investigations, the analysis of cross sections and the moistening of the weathered limestone have led me to a conclusion that the flow of water is, in the case of thick weathered zones on the passage walls, effected molecular diffusion and capillary action, which proves to be the faster. In the cases I am describing, the rock is not only dissolved on the surface, that is frontally, and it does not leave in its wake the smoothed surfaces of the cave walls, as is the case when it is in contact with flowing water where this contact between water and rock lasts sufficiently long to bring about the chemical reaction.
When the inflow or outflow of water stops and the solution which is present in the connected pores gets saturated, or dries up, the rock cease to be dissolved. The weathering of carbonate rocks is usually caused by dissolution, that is, by the transition from rock into solution (Summerfield 1991). The dissolution is distinctly selective. In the first place, smaller grains and the contacts among grains are dissolved. When dealing with clastic sedimentary rocks Skaberne (1980) noted, that they weather chemically and crumble in relation with their structure. I noticed the similar phenomenon also in limestones and dolomites, especially when the dissolution along the edges of mineral granules weakens the mechanical cohesion of the rock. The fact that limestones with sparite and microsparite structure start dissolving along the edges of grains and along the deformities in the crystal surface is already known from the literature (Ford and Williams 1989).
During dissolution pores get larger and become more and more interconnected, so that aggressive water advances more easily and deeper into the rock's interior. With the lapse of time even the more resistant parts of carbonate rock weather as well. Calcite veins and the shell fragments that jut out from the weathered rock surface become porous and soft. During further dissolution the rock gets more porous and fragile until its structure completely collapses. The flow of water in all these cases is not large and the actual significance may be ascribed to the moisture; this moisture is well capable to cause dissolution. The term corrosive moisture used by Davis and Mosch (1988), when they describe the weathering of the clay pebble surfaces, denotes condensed or vadose (percolation) water.
The humidity of weathered walls changed significantly during the course of year. Sometimes the walls were completely wet, at another time entirely dry. The fluctuation of humidity during the year is quite obvious and is related both to the precipitations as well as to the velocity of the trickling along the walls. The penetration of moisture into the weathered part of the wall is very fast. It may stop only at the larger calcite veins, and it may also take some time to cross over open cracks or those partly filled with clay. I attempted to explain the formation of mosaic porosity and the sponge-like rock structure by the model, which is similar to that used by Trudgill (1985), when he tried to determine the formation of porosity in the limestone karst soil (rendzina). Following my own observations, I presume that in cases when the wall dries up, the new moisture penetrates into the rock even faster, because the pores have been emptied. Consequently, I believe that dissolution is going on faster as well, so that water in pores loses its aggressiveness again, the dissolution becomes saturated and pH increases. At every new water wave the water will use the pores and channels which were produced during the previous cycle. It may also widen them a little and the main dissolution front will thus, with each new water wave, move deeper into the rock. Through its contact with carbonate particles in the porous skeleton the aggressive water quickly becomes saturated; so that only a part of its former quantity is still able to interact with the rock. The mechanism is repeated in cycles, so that the rock becomes ever more porous and does not dissolve entirely at first.
This incomplete limestone dissolution is most likely taking place in the vadose zone. The flow of water in the phreatic zone would wash the particles from the wall - if not before, then during its retreat, or the silt would crumble away from the wall of its own accord. The water flow may also simultaneously carry away the ions, and the dissolution would progress into the depth slower, for it would act frontally. There may exist a possibility that the incomplete dissolution is, nevertheless, taking place, yet because of the continuous washing away of the particles we are unable to recognize it. Limestone porosity in the given cases increased because calcite grains that were less resistant to dissolution got dissolved. Dissolved primarily were those grains that were smaller and those whose structure was less well ordered due to the presence of Mg ions in their crystal lattice. Mg ions are, owing to their lower ionic potential, more mobile and are the first to leave their places in the crystal lattice thus they further weaken the interior structure and increase the proneness to dissolution. Theoretically, water in porous media in contact with calcite reach equilibrium immediately it becomes saturated or even supersaturated against CaCO3, but it is not necessary that it is also saturated against Mg2+, so the dissolution of the parts containing Mg ion may go on (Bathurst 1975).
The decrease of the Mg ion share in the weathered parts of limestones and dolomites has already been observed by several authors. The fact that Mg is being extracted from limestone during dissolution was found out also by Kogovšek and Habič (1981) in their measurements of the magnesium hardness. The reason for this is the greater solubility product of MgCO3 when compared to CaCO3. The leaching of Mg during dolomite weathering was stated also by Burger (1989), yet he did not try to explain it. Slabe (1988) interprets the lower share of Mg on the surface of the cave wall which has already been dissolved by corrosion, as the complete dissolution of impure limestone which contains Mg and as the precipitation of pure calcite crystals from the condensed moisture.
Yet, in all the cases, I am describing here, we are actually not witnessing the calcite crystal extraction but the weathering of limestones and dolomites. In cases when the weathered rock is in direct contact with the cave environment, a thin calcite crust is almost always being extracted and deposited on its surface. During the rock's drying, the saturated moisture oozes out of its pores, or it is squeezed towards the weathered rock's surface by the incoming water. However, it still remains somewhat unclear, where do the dissolved ions in these cases actually migrate. Especially when we take into account that the weathered rock which is in contact with the alluvium is not even covered with a flowstone layer and that we have not yet found any minerals containing Mg.
The answers to the questions are:
- The zones of carbonate silt or clay and white porous rocks on the cave passage walls are a product of weathering processes and not of secondary minerals precipitation.
- Dissolution penetrates into the rock along various structures, such as cracks, primary porosity, microstructures, crystal's deformities and primary structures covered by micritization or neomorphism, which may also hinder the expansion of dissolution.
- Selective dissolution forms the coarseness on the surface and the sponge-like structure of the weathered rock, which may reach a depth of several centimetres.
My opinion is that the occurrence of the incomplete dissolution of carbonate rocks within speleogenesis may represent an important factor for the formation of initial channels, because carbonate rock porosity increases proportionally with the selective dissolution of calcite and dolomite by carbonic acid and not with dissolution of the more soluble additions (gypsum, anhydrite, etc.). During the weathering, pores in limestone and dolomite augment, they establish connections among them, which leads to increased effective porosity. The enlargement of pores and the expansion of their interconnections consequently leads to the formation of initial channels. Incomplete dissolution accompanied by the simultaneous washing away of the weathered rock also accelerates the growth of passages. By means of this process the passage's enlargement is faster and more intensive especially during floods or high water splash through the cave channels.
References
Bathurst R. G. C. 1975. Carbonate Sediments and Their Diagenesis. Second enlarged edition, Amsterdam: Elsevier, 658 pp,.
Burger D. 1989. Dolomite weathering and micromorphology of paleosoils in the Franconian Jura. Catena Supplement 15, Cremlingen, 261-267,.
Davis G. D. and Mosch C. 1988. Pebble indentations: A New Speleogen from a Colorado Cave. National Speleological Society Bulletin 50, 17 – 20.
Dreybrodt W. 1988. Processes in Karst Systems. Berlin, Heidelberg: Springer-Verlag, 288 pp.
Ford D. C. and Williams P. W. 1989. Karst Geomorphology and Hydrology. London: Unwin Human, 601 pp.
Kaufmann O., Bini A., Tognini P. and Quinif Y. 1999. Etude Microscopique d’une latérite de type fantôme de roche. Karst 99: colloque européen: des paysages du karst au géosystème karstique: dynamiques, structures et enregistrement karstiques (Etudes de géographie physique, supplément 28), 129 – 133, Aix-en-Provence.
Kogovšek J. and Habič P. 1981. Preučevanje vertikalnega prenikanja vode na primerih Planinske in Postojnske jame. Acta carsologica 9, 129 -148.
Mihevc A. 1996. Brezstropa jama pri Povirju. Naše jame 38, 65-75.
Palmer A. N. 1981. Geology of Wind Cave. Wind Cave National Park, South Dakota. Hot Springs: Wind Cave Natl. Hist. Assoc., 44 pp.
Pezdič J., Šušteršič F. and Mišič M. 1998. On the role of clay-carbonate reactions in speleo-inception; A contribution to the understanding of the earliest stage of karst channel formation. Acta carsologica 27 (1), 187-200.
Renault P. 1968. Contribution à l’étude des actions mécaniques et sédimentologiques dans la spéléogenèse (Troisième partie). Annales de Spéléologie, Centre National de la Recherche Scientifique 23 (3), 530-596, Moulis.
Skaberne D. 1980. Predlog klasifikacije in nomenklature klastičnih sedimentnih kamnin.- 1.del, Predlog granulometrijske klasifikacije in nomenklature. Rudarsko metalurški zbornik 27 (1), 21- 46, Ljubljana.
Slabe T. 1988. Kondenzna korozija na skalnem obodu Komarjevega rova v Dimnicah. Acta carsologica 17, 79-92.
Slabe T. 1999. Subcutaneous rock forms. Acta carsologica, 28 (2), 255-271.
Summerfield M.A. 1991. Global geomorphology, an introduction to the study of landforms. New York: John Wiley & Sons, 537 pp.
Trudgill S. T. 1985. Limestone Geomorphology. London and New York: Longman, 196 pp.
Vergari A. and Quinif Y. 1997. Les paleokarst du Hainaut. Geodinamica Acta 10 (4), 175-187.